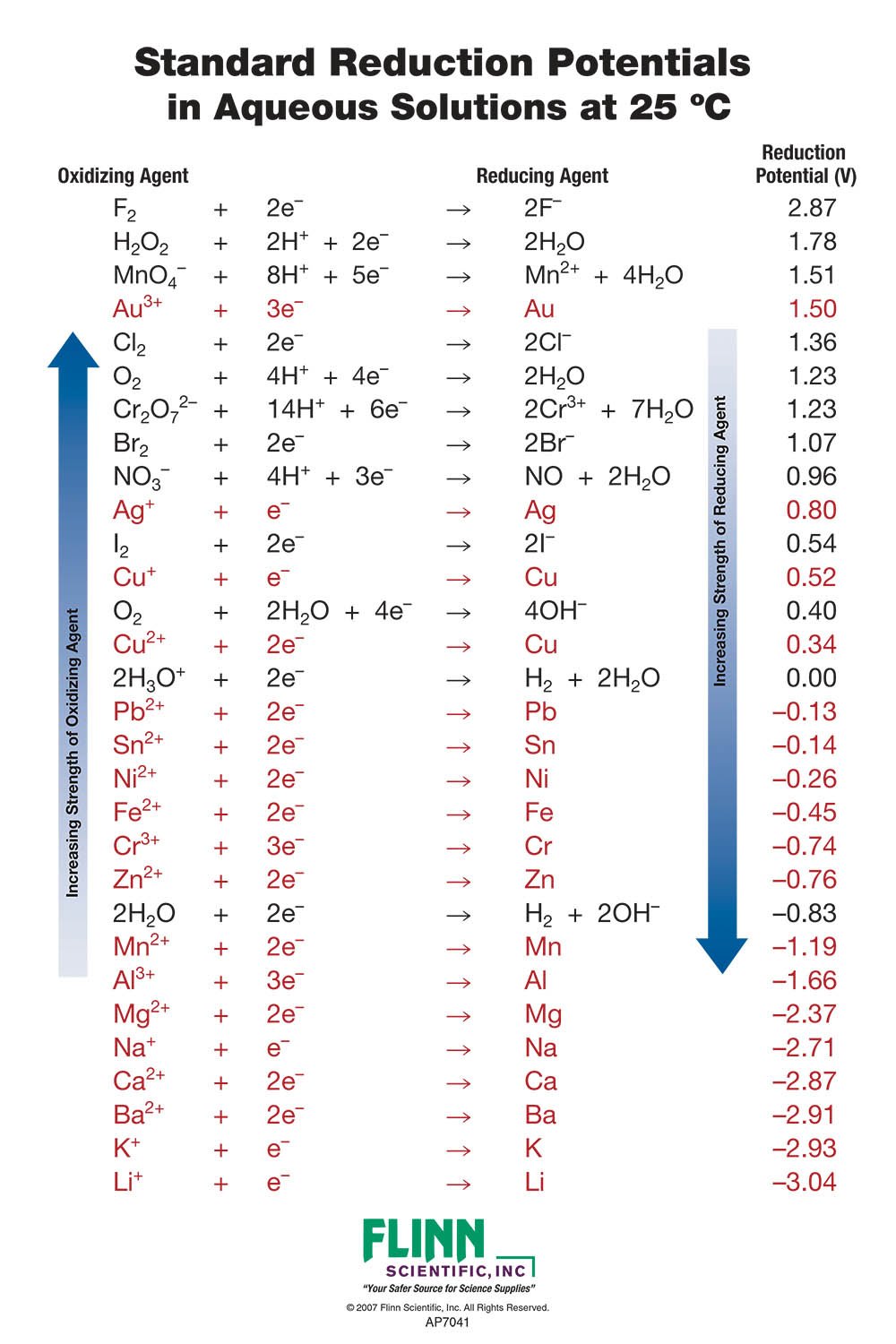
Standard Cell Potential Spontaneous . by the end of this section, you will be able to: calculate cell potentials and predict redox spontaneity using standard electrode potentials. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. Unlike the spontaneous oxidation of copper by aqueous silver. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the positive value for the standard cell potential indicates the process is spontaneous under standard state. calculate cell potentials and predict redox spontaneity using standard electrode potentials.
from notes.pegas.is
calculate cell potentials and predict redox spontaneity using standard electrode potentials. by the end of this section, you will be able to: calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. Unlike the spontaneous oxidation of copper by aqueous silver. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the positive value for the standard cell potential indicates the process is spontaneous under standard state. Describe and relate the definitions of electrode and cell potentials.
Types of Reactions Pega's Notes
Standard Cell Potential Spontaneous the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. calculate cell potentials and predict redox spontaneity using standard electrode potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the positive value for the standard cell potential indicates the process is spontaneous under standard state. Unlike the spontaneous oxidation of copper by aqueous silver. Describe and relate the definitions of electrode and cell potentials. calculate cell potentials and predict redox spontaneity using standard electrode potentials. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. by the end of this section, you will be able to: 1 atm for gases, 1 m for solutions, and the pure solid for.
From www.youtube.com
Practice with Redox Spontaneity and Standard Cell Potential OpenStax Standard Cell Potential Spontaneous calculate cell potentials and predict redox spontaneity using standard electrode potentials. calculate cell potentials and predict redox spontaneity using standard electrode potentials. the positive value for the standard cell potential indicates the process is spontaneous under standard state. Describe and relate the definitions of electrode and cell potentials. Unlike the spontaneous oxidation of copper by aqueous silver.. Standard Cell Potential Spontaneous.
From www.youtube.com
Electrochemistry 05 Calculating Standard Cell Potentials YouTube Standard Cell Potential Spontaneous 1 atm for gases, 1 m for solutions, and the pure solid for. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Unlike. Standard Cell Potential Spontaneous.
From www.numerade.com
Determine the overall reaction and its standard cell potential at 25 °C Standard Cell Potential Spontaneous the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. by the end of this section, you will be able to: calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for. Standard Cell Potential Spontaneous.
From www.numerade.com
SOLVED Based on the sign of the standard cell potential, Ecell Standard Cell Potential Spontaneous calculate cell potentials and predict redox spontaneity using standard electrode potentials. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the positive value for the standard. Standard Cell Potential Spontaneous.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Spontaneous calculate cell potentials and predict redox spontaneity using standard electrode potentials. the positive value for the standard cell potential indicates the process is spontaneous under standard state. Describe and relate the definitions of electrode and cell potentials. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous. Standard Cell Potential Spontaneous.
From www.numerade.com
SOLVED Title Standard Cell Potentials and Spontaneity of Redox Standard Cell Potential Spontaneous the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. the positive value for the standard cell potential indicates the process is. Standard Cell Potential Spontaneous.
From www.chegg.com
Solved Based on the sign of the standard cell potential, Standard Cell Potential Spontaneous 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the positive value for the standard cell potential indicates the process is spontaneous under standard state. Unlike the spontaneous oxidation of copper by aqueous. Standard Cell Potential Spontaneous.
From www.numerade.com
SOLVED Title Standard Cell Potentials and Spontaneity of Redox Standard Cell Potential Spontaneous the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. calculate cell potentials and predict redox spontaneity using standard electrode potentials. Unlike the. Standard Cell Potential Spontaneous.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Spontaneous Unlike the spontaneous oxidation of copper by aqueous silver. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. the potential of the cell under standard. Standard Cell Potential Spontaneous.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Spontaneous Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. calculate cell potentials and predict redox spontaneity using standard electrode potentials. by the end of this section, you will be able to: the standard cell potential (e°cell) is measured at. Standard Cell Potential Spontaneous.
From www.chegg.com
Solved For each reaction listed, determine its standard cell Standard Cell Potential Spontaneous 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Unlike the spontaneous oxidation of copper by aqueous silver. calculate cell potentials and predict redox spontaneity using standard electrode potentials. by the end of this section, you will be able to: the positive value for the standard cell potential indicates. Standard Cell Potential Spontaneous.
From www.pveducation.org
Standard Potential PVEducation Standard Cell Potential Spontaneous Unlike the spontaneous oxidation of copper by aqueous silver. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the positive value for the standard cell potential indicates. Standard Cell Potential Spontaneous.
From www.chegg.com
Solved 5. Calculate the standard cell potential for the Standard Cell Potential Spontaneous the positive value for the standard cell potential indicates the process is spontaneous under standard state. by the end of this section, you will be able to: calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. Unlike the spontaneous oxidation of copper. Standard Cell Potential Spontaneous.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Cell Potential Spontaneous the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. 42 rows calculate the standard cell potential of a voltaic cell that. Standard Cell Potential Spontaneous.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Cell Potential Spontaneous Unlike the spontaneous oxidation of copper by aqueous silver. calculate cell potentials and predict redox spontaneity using standard electrode potentials. calculate cell potentials and predict redox spontaneity using standard electrode potentials. by the end of this section, you will be able to: the potential of the cell under standard conditions (1 m for solutions, 1 atm. Standard Cell Potential Spontaneous.
From f15.beauty
Standard Reduction Potential Table Standard Cell Potential Spontaneous the positive value for the standard cell potential indicates the process is spontaneous under standard state. Unlike the spontaneous oxidation of copper by aqueous silver. calculate cell potentials and predict redox spontaneity using standard electrode potentials. calculate cell potentials and predict redox spontaneity using standard electrode potentials. 42 rows calculate the standard cell potential of a. Standard Cell Potential Spontaneous.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Spontaneous Describe and relate the definitions of electrode and cell potentials. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous oxidation of copper by aqueous silver. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. calculate cell potentials and predict. Standard Cell Potential Spontaneous.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Spontaneous Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. calculate cell potentials and predict redox spontaneity using standard electrode potentials. Unlike the spontaneous oxidation of copper by aqueous silver. the potential of the cell under standard conditions (1 m for. Standard Cell Potential Spontaneous.